Sustainable hydrogen from sunlight

In the search for energy sources of the future that avoid CO2 emissions and still provide a stable energy supply, hydrogen has a key role to play. For this reason, a tandem module is being developed within the framework of the Fraunhofer joint project ”Neo-PEC”, which in future will generate green hydrogen directly by means of sunlight in a cost-effective and clean manner and thus enable a decentralized hydrogen supply.

Solar water splitting
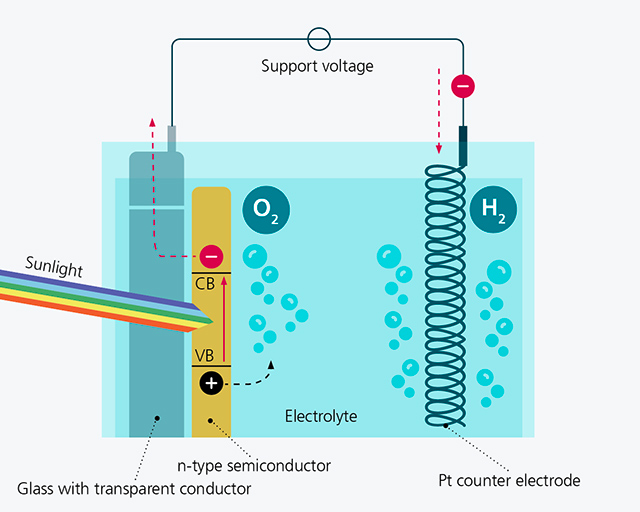
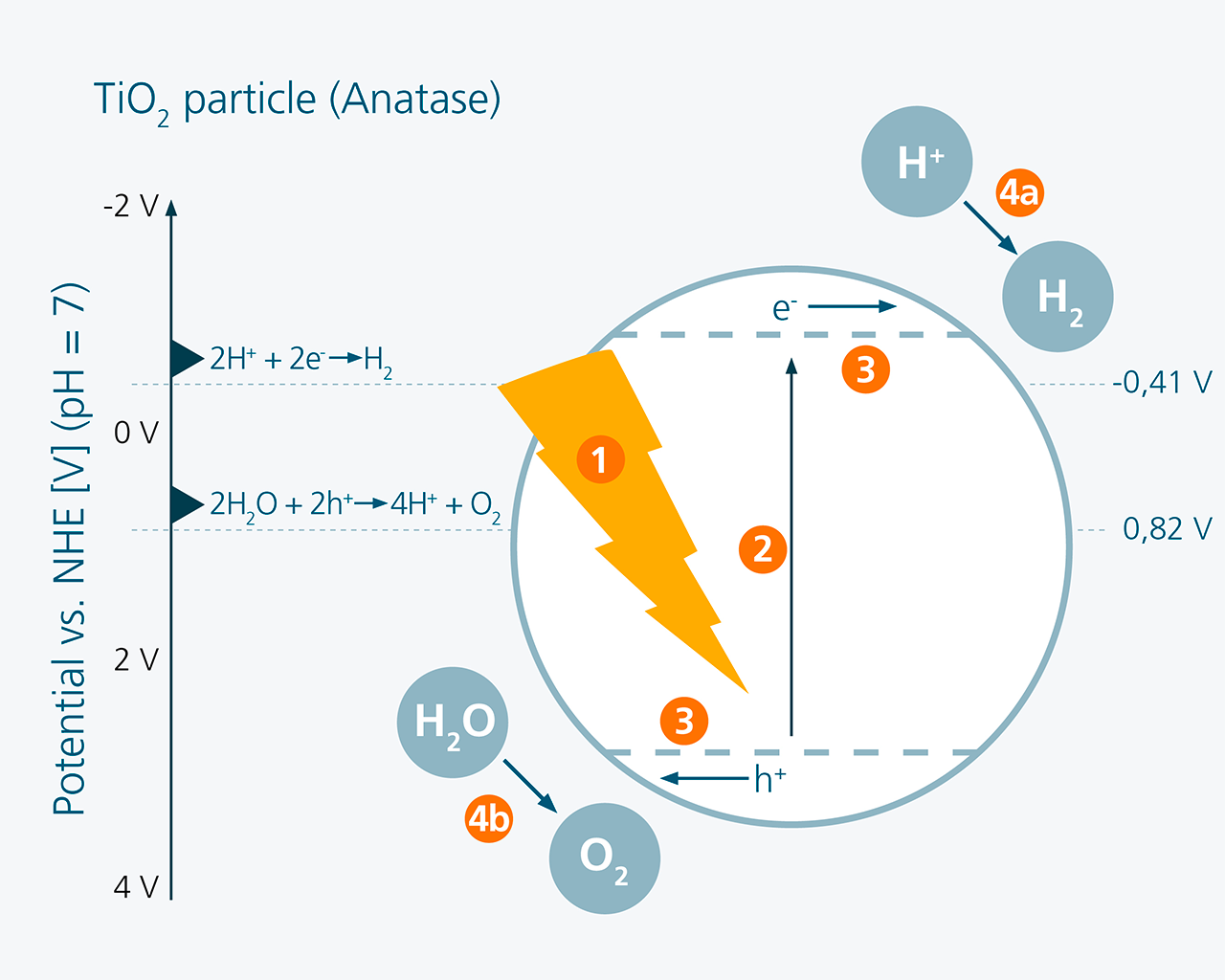
The underlying principle has been known for more than 40 years and is similar to natural photosynthesis: Photo Electro-Catalytic PEC (water splitting) is a light-driven water electrolysis in which electrons and holes are first generated by sunlight in a suitable semiconducting absorber material. In the simplest case, this can be achieved with titanium oxide particles in an electrolyte, for example (see diagram below, left). The electrons and holes energetically raised by light then reach the interface of the particle to the aqueous electrolyte by diffusion and/or band bending and drive a chemical reaction there for hydrogen and oxygen formation respectively.
The graphic in the middle shows photocatalytic water splitting using an illuminated n-type semiconductor layer for oxygen production and a platinum counter electrode for hydrogen production. The advantage over the variant with particles is that the water and oxygen production already take place spatially separated. This setup, which is also referred to as a half cell, usually also requires a small external auxiliary voltage to further increase the energy of the electrons and holes.
Project approach
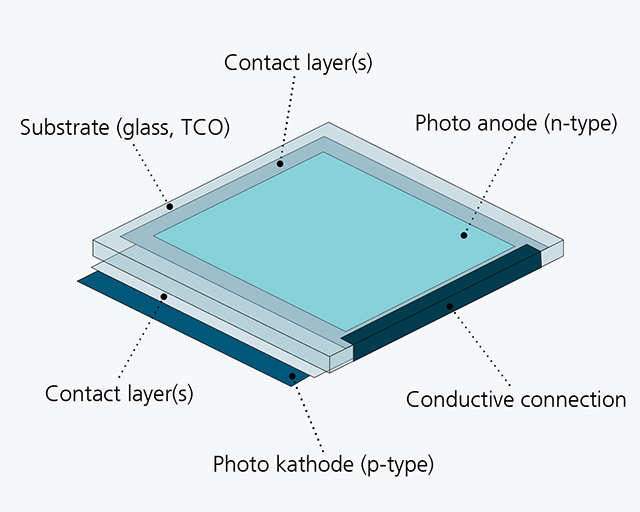
The approach chosen in the joint project ”Neo-PEC” envisages the realization of a tandem cell consisting of two half-cells (see figure below, right), which allows the gases produced in the process to be discharged separately.
With this type of cell, a maximum efficiency of photocatalytic water splitting of more than 25 percent is theoretically possible1, since the semiconductors each use a different part of the solar spectrum. There is an analogy here with natural photosynthesis, which also uses two areas of sunlight, the blue and red color components. In practice, however, only about one percent efficiency is currently achieved for such simple tandem cells. The reasons for this are manifold:
- Insufficient quality of the semiconductors
- Highly absorbent contact structures
- Rapid degradation in the electrolyte and losses due to mismatched half cells
On the other hand, 19 percent efficiency has already been demonstrated with a complex structure, technically complex deposition processes and expensive materials2.
This is where the project comes in. The aim is to close the gap between the 19 percent already achieved and the one per cent, while maintaining a structure as simple as possible. To achieve this, the entire system is being optimized: The many years of know-how of the Fraunhofer IST will be used for the transparent contacts and adapted to the requirements of the tandem cell. High-quality semiconductors with intrinsic durability are to be realized at the Fraunhofer IST using novel and modified PVD processes which permit large-area, low-defect and low-cost deposition.
The project is being implemented in close cooperation with the Fraunhofer Institute for Ceramic Technologies and Systems IKTS and the Fraunhofer Center for Silicon Photovoltaics CSP, which contribute expertise in the areas of sputtering target production, thermal treatment, photoelectric characterization and large-scale demonstrator construction.
Results
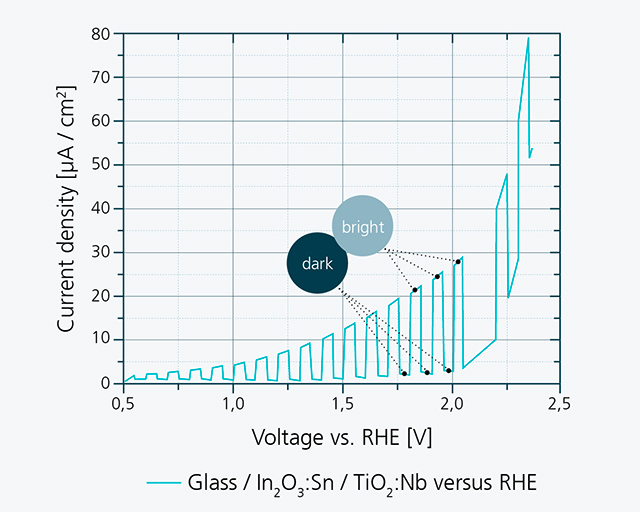
In the first phase of the project, n-type half cells based on titanium oxide were realized at the Fraunhofer IST by means of sputter deposition and subsequent "explosive growth" as a model system for test purposes (see figure below left). The measurement of the photocurrents in the aqueous electrolyte was carried out at the Fraunhofer CSP (see figure below right). The current difference between light and dark conditions proves the desired effectiveness as a photoanode and the associated photoelectric oxygen generation. In addition, for voltages above about 1.5 volts, the conventional voltage-driven electrolysis can be recognized by the strongly increasing current. Overall, however, the photogenerated currents of 10 to 20 µA/cm2 are still low compared to the targeted values in the milliampere range.

Outlook
In the further course of the project, there will be a transition to tungsten oxide as an n-type material, which allows higher photocurrents than titanium oxide, and the establishment of copper chromium oxide (CuCrO2) as a p-type cathode for hydrogen production. Together with the project partners, weak points are identified and the photocurrent and efficiency are increased on this basis. The goal is a demonstrator module with an area of 1 m2 by the end of the project.
The project
The project was funded by the Fraunhofer-Gesellschaft in the context of internal programs (PREPARE).
Literature:
1 Montoya et al., Materials for solar fuels and chemicals, Nature Materials 16 (2017) 70–81.
2 Cheng et al., Monolithic Photoelectrochemical Device for Direct Water Splitting with 19% Efficiency, ACS Energy Lett. 3 (2018), 1795–1800.
This article is part of the Annual Report 2021.