Closed-loop electrochemical processes for the extraction of pure elements from lunar regolith

An important prerequisite for a long-term and sustainable human presence on the moon is the availability of resources such as pure metals and oxygen in order to construct, for example, building accommodation, a research station and the necessary infrastructure for astronauts. The so-called lunar regolith, loose rock found on the surface of the moon, consists of metal oxides such as iron, titanium, aluminum and magnesium. In these oxides, oxygen is present in a bound form, thereby accounting for around 50 percent of the total mass. In order to make both the oxygen and the metals in their pure forms available to humans, a process under space conditions without consumable materials is necessary. In collaboration with the Institute of Space Systems (IRAS) at the TU Braunschweig, the Fraunhofer IST is working on a procedure for extracting pure elements from lunar regolith, considering the conditions prevailing on the moon.


Electrochemical deposition of pure metals from regolith
As a result of the lack of genuine lunar regolith (the Apollo and Luna missions only brought around 360 kg of the material back to Earth), so-called regolith simulants – such as the European Astronaut Centre lunar regolith simulant 1 (EAC-1A) or the Johnson Space Center-developed lunar simulants JSC-1A and JSC-2A – must be utilized for the development of processing technologies. The goal is to extract metals and oxygen from regolith (or rather the simulants). Ionic liquids are the solvent of choice. They can be used not only to dissolve oxides but also to produce electrochemically oxygen and even metals such as aluminum, titanium and magnesium as well as silicon, that are not accessible in aqueous media.
Advantages of ionic liquids as solvents
Ionic liquids are organic salts which are liquids at temperatures below 100 °C. They have negligible vapor pressure and do not evaporate in a vacuum. Consequently, no risk of material loss or environmental contamination exists – even on the moon. As ionic liquids are often sensitive to moisture, special safety precautions must be taken when processing them on Earth, for example the application of dry inert gases such as nitrogen or argon. This is not necessary on the moon, as a vacuum is present there. As a result, moisture is not a problem, and the process control is therefore simplified enormously.
The project
The aim of the “ELMORE” project is the dissolution of regolith by means of modern electrochemical procedures within a closed-loop system and the deposition of the desired pure metals as well as the capture of the oxygen generated thereby. In a first process step, the regolith simulants produced by IRAS are chemically dissolved in ionic liquids and the metal ions are subsequently converted into the corresponding pure metals. The electrochemical deposition of the metals thereby takes place at the cathode. Simultaneously, oxygen is generated at the anode.
Results
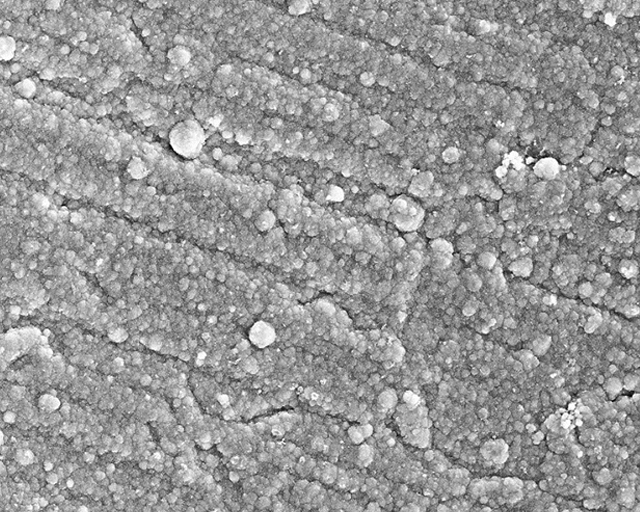
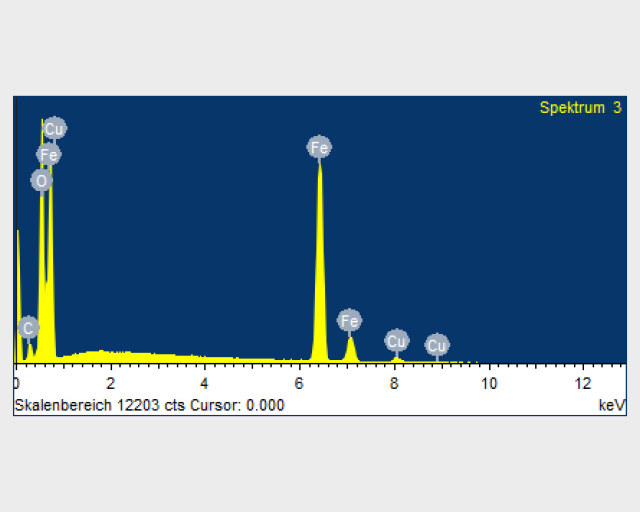
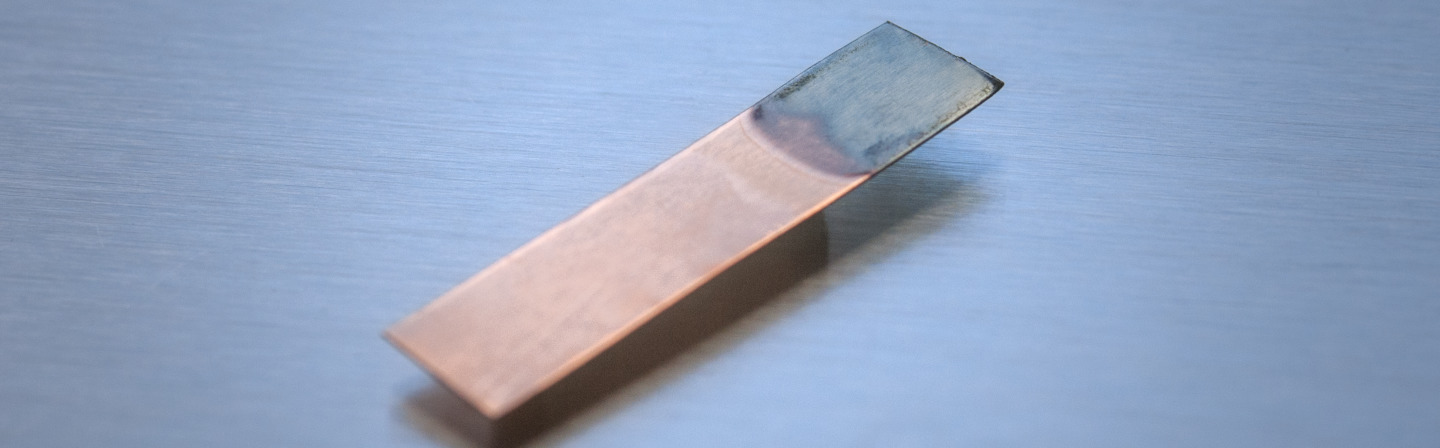
For a proof of concept, iron oxide as a component of regolith was chemically dissolved in ionic liquids and subsequently electrochemically deposited on a copper substrate at 100 °C (see Figure above). The Figure below on the left below on the right shows the SEM image of the iron deposition on the copper substrate. The Figure below on the right shows the EDX profile of the iron layer deposited on the copper. This iron layer did not contain any impurities except oxygen as a result of rapid oxidation of iron in air and formation of iron oxide.
Outlook
The available results represent solely the simple metal-oxide dissolution and its electrochemical extraction. In a next step, the proof of concept is to be transferred to simulants of lunar regoliths. At the Fraunhofer IST, investigations are being carried out into how metal oxides dissolve at various temperatures in differing ionic liquids. Subsequently, regolith is to be dissolved in ionic liquids. Further experiments are planned in which metals such as aluminum and iron will be deposited potentiostatically or galvanostatically at the cathode, whilst oxygen is released at the anode. The advantage of this procedure is the transfer to processes that are environmentally friendly; the terrestrial production of e. g. iron or aluminum is a process which emits large quantities of CO2. The energy demand for the lunar process of aluminum extraction is around 7 kWh/kg aluminum. In comparison, terrestrial procedures require 15 kWh/kg aluminum as a result of the upstream smelting process.
This article is part of the annual report 2020.