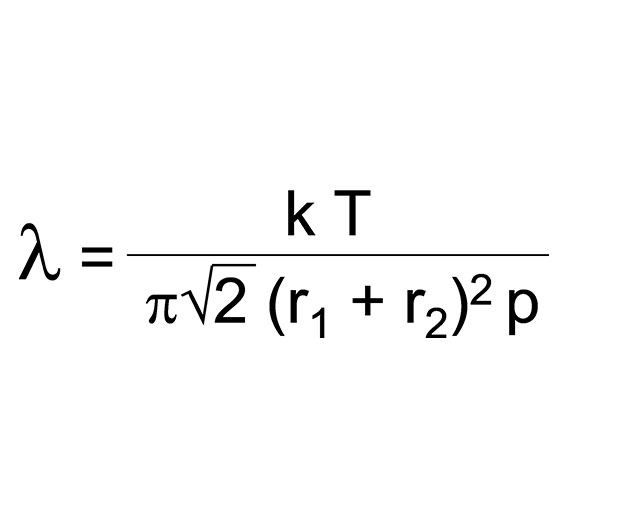What is actually ”vacuum”?
At Leybold-Heraeus, at that time a market leader in vacuum plant construction, I learned the following ambiguous statement: “We’ve got a vacuum in our heads!” The ancient Greeks had already been involved with vacuums in around 500 BC. Nowadays, physicists define a vacuum strictly as a space containing neither matter, radiation nor force fields. In order to make use of the vacuum technically, for instance to deposit high-quality thin films, we definitely need matter as well as electrical and magnetic fields, and, depending on the circumstances, radiation too. We use highly rarefied gases to operate low-pressure plasmas, which in turn serve as an energy source during the depositing of coatings.
It is the principle employed by the PVD (Physical Vapor Deposition) or PACVD (Plasma-Assisted Chemical Vapor Deposition) processes to generate the layer-forming atoms or molecules at location A and to transport them over a certain distance in the macroscopic range (from a few centimeters to several decimeters) to location B where they form a layer on the substrate. One decisive factor here is the “mean free path length λ“.This indicates the average distance that a particle (an electron, atom or molecule) can travel before it collides with another particle. We will return to this later.
We will return to this later. First, we would like to take a brief look at the pressure. DIN 1314 defines the pressure p of fluids (gases and liquids) as the quotient of the normal force on a surface area and the content of this surface. The corresponding SI unit is:
1 bar = 1,000 mbar = 105 N/m2 = 105 Pa
In technical applications, this is often derived from the partial pressure pi or the total pressure pt. The partial pressure of any given gas is the pressure that this gas would have if it were present in a vessel on its own. The total pressure in a vessel is the sum of the partial pressures of all the gases contained in it. And last but not least, we know the standard pressure pn or the atmospheric pressure from the weather forecasts.According to DIN 1343, this is:
pn = 1.013,25 mbar = 1.013,25 hPa
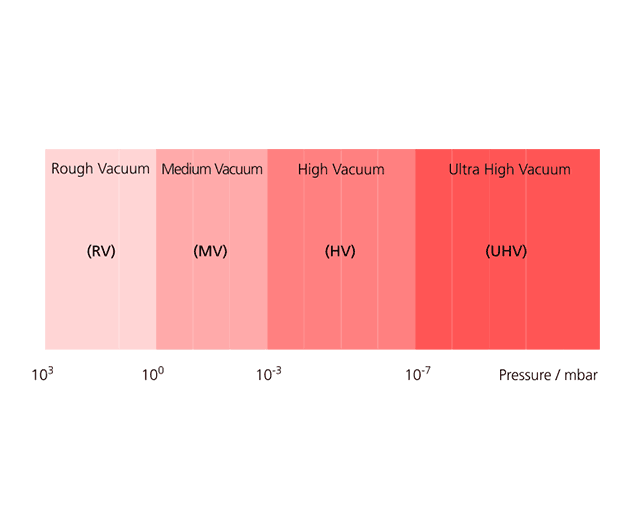
Vacuums that we can produce on earth with the appropriate technical effort comprise about 15 orders of magnitude of pressure. The adjacent figure shows the corresponding pressure ranges. Pressures below 10-10 mbar are more interesting for collision experiments with high-energy particles in accelerators; the most important vacuum coating processes take place at pressures ranging from 10-1 to 10-4 mbar.
Pumping a gas out of a vessel with a fixed volume serves reduces the particle density and so increases the mean free path length. In other words: “It clears the path”. This mean free path length is a function of the absolute gas temperature T and the impact radii r1 and r2 of the two partners involved, but above all, as can easily be seen, it is inversely proportional to the pressure. The explicit formula is shown on the left. Simplified, this means: pλ = const, the constant contains the values T, r1 and r2.
The following table shows particle densities and mean free path lengths for different “gaseous atmospheres”:
| environment | Edge length of a cubic vessel | Particles contained in the vessel | Mean free path length of the particles |
|---|---|---|---|
| Air at atmospheric pressure | 0.001 mm | 30,000,000 | 0.0000066 mm |
| Air on Mount Everest | 0.001 mm | 9,000,000 | 0.00002 mm |
| Orbit of the Space Shuttle | 0.1 mm | 1,000 | 1.68 km |
| Interstellar space | 1 cm | 3 | 670,000,000 km |
| Intergalactic space | 10 m | 1 | 180,000 light years |
Particles in the air surrounding us can therefore only move an average of 66 nm before they collide with another particle, while particles in deep space lead a relatively lonely life. The following simple equation can be used to provide an estimate, it is often sufficiently accurate:
pλ = 5 . 10-5 mbar . m
For a coating process being performed at a pressure of 10-2 mbar, this results in an average free path length of 5 mm. Good coating systems that are sealed and whose inner surfaces are clean and dry, achieve final pressures of around 10-6 or 10-7 mbar before the process gas is introduced. Such values are necessary to remove reactive gases such as oxygen, nitrogen or the water vapor that is always contained in atmospheric air as completely as possible and so to achieve extremely high layer qualities.
Just how vacuums are generated and which factors play a role in this will be clarified in the next article.
Last modified: