Innovation through universities and research institutions



Challenge
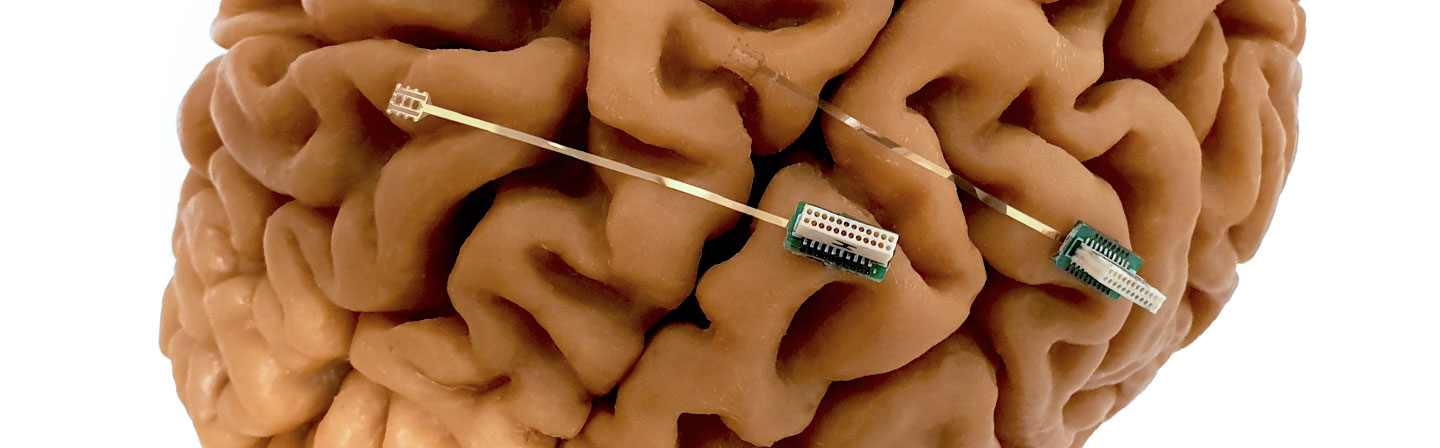
Medical-technology innovations from SMEs often fail due to the implementation of special processes and the transfer from basic research to the market. In contrast, expensive machines in research institutions are not sufficiently utilized. The “TransPlaMed” platform is therefore intended to optimize the transfer of technology and knowledge for special processes in medical technology.
Solution
For the joint project, the Fraunhofer IST is providing a coating facility for the validation of coating processes for medical products in accordance with DIN EN ISO 13485, DIN EN ISO 10993 and DIN EN ISO 14971 for shared use. The facility is suitable for prototype development in science and the industrial production of medical products. It consists of an atomic layer deposition unit for which an overall quality and risk-management system has been designed and implemented, and has been validated for coating processes for medical products.
Added value
The joint project fulfils the priorities defined in the associated regional innovation strategy in Lower Saxony, as it clearly addresses the specialization fields of the healthcare industry, new materials and production technology, as well as the digitalization of the economy in Industry 4.0.
