
Palladium gas separation membranes

The demand for clean and green energy has meant a continuous increase in the consumption of hydrogen in recent years. An economically efficient production of small and medium quantities of hydrogen can be achieved with the help of thin palladium membranes applied to porous pipe surfaces. The Pd membranes are selectively permeable to hydrogen, thus allowing efficient separation of hydrogen from a gas mixture. Under a cooperation agreement, the Fraunhofer IST is working together with the Plansee SE and Linde AG companies in this field.

Principle of hydrogen separation
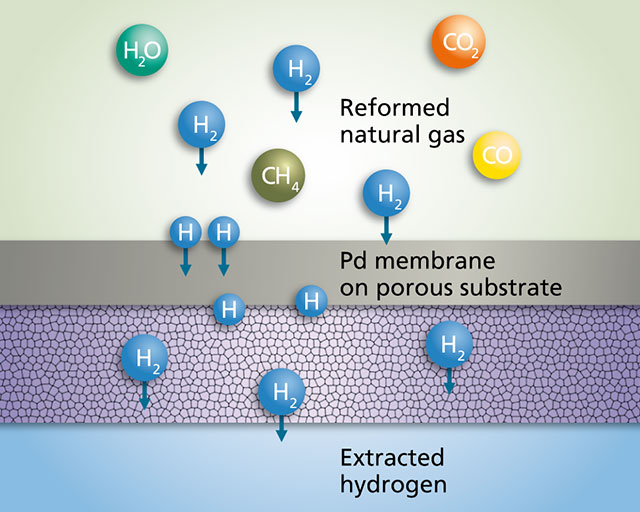
In some elements, such as palladium, vanadium, nickel or iron and in alloys such as Ag-Pd, Ni-Zr, hydrogen is highly soluble and has high diffusivity at elevated temperatures. If the metallic membrane structure is sufficiently dense and thus only permeable to hydrogen atoms, a system of this kind can be used for separating H2 from a gas mixture. To avoid corrosion, membrane structures made of noble elements such as palladium are preferably used. Given the high cost of pure palladium and due to the fact that the hydrogen flow rate is inversely proportional to the thickness of the membrane, only very thin Pd membranes about 10 µm thick are used, which are deposited on a relatively thick (about 1 mm) porous substrate.
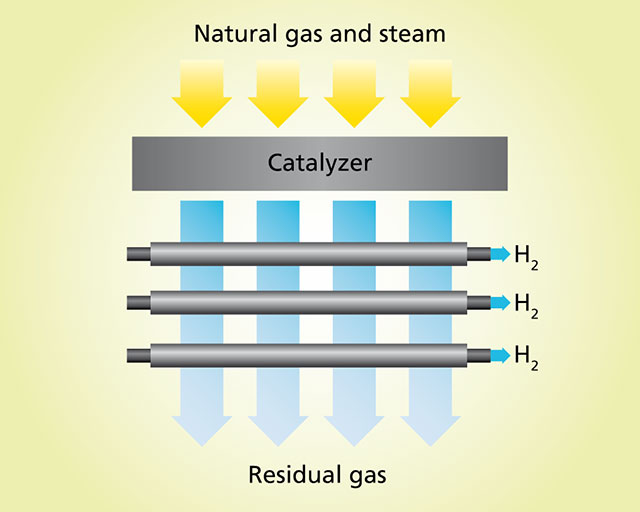
Hydrogen may be produced via steam reforming of natural gas. Here steam is added to natural gas and this mixture passed over catalyst particles. In an endothermic reaction at operating temperatures of approximately 600 °C, hydrogen molecules and carbon oxides are produced. When this gas mixture meets a palladium surface, the hydrogen permeates through the metal and can be extracted on the other side for further processing.
Gas-separation membrane tubes
Under the trade name ITM our partner Plansee SE produces by powder metallurgy thin tubes made of an Fe-Cr alloy. With a porosity of approximately 40 vol.-% they are highly gas-permeable and form the substrate material for the thin Pd membranes which the Fraunhofer IST applies by means of a PVD process. In addition, between the ITM tubes and the Pd membrane there is a porous ceramic diffusion barrier of zirconium oxide (ZrO2), stabilized with yttrium oxide (Y2O3). This membrane prevents Pd diffusion into the Fe-Cr substrate, thus ensuring the long-term stability of the coating. The leakage rate of the Pd membrane essentially determines the performance of the system and should be as low as possible. However, the thin Pd coatings have nodular defects. This reduces the impermeability of the membrane so that not only H2 but also other molecules can diffuse through it. To prevent this, these flaws were sealed over by a second electroplated layer of palladium.
This article is part of the annual report 2015.